Did you know that the majority of the “metals” we use aren’t actually metals at all? Rather than metals, these materials are alloys and they are everywhere around us! From dental fillings to aircraft, alloys are a large part of our day-to-day lives. Learn what exactly an alloy is and how they’re made here.
What Is Metal?
A metal is the pure chemical element, like you would find on the periodic table. 91 of the 118 elements on the periodic table are metals – which makes them some of the most plentiful elements in the world.
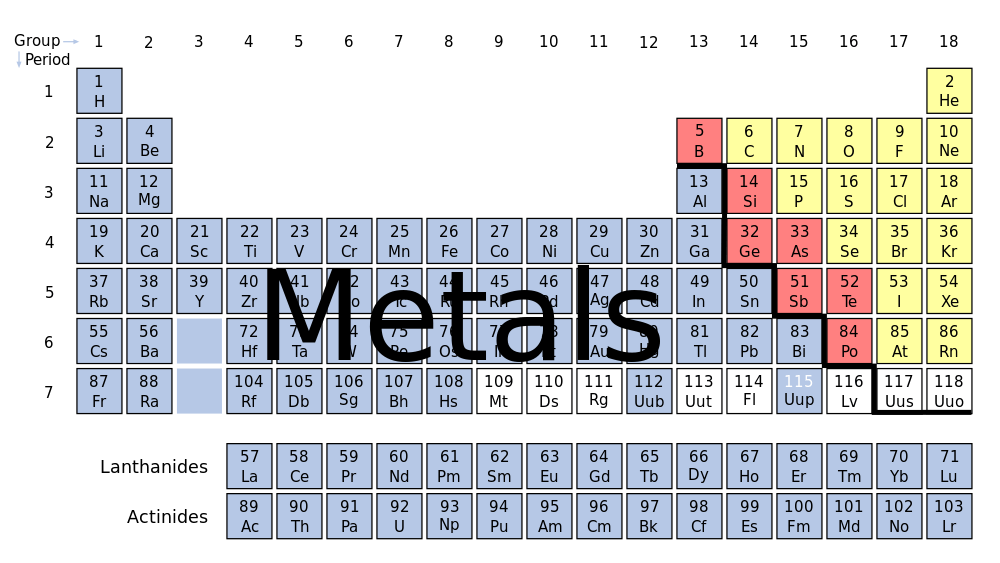
On the periodic table, all elements are divided into metals and nonmetals. What makes something a “metal” is that it occurs naturally in nature, has a luster, are good conductors of heat and electricity, and are much denser than non-metals.
There are 5 main categories of metals:
- Base metals
- Ferrous metals
- Noble metals
- Precious metals
- Heavy metals
Base Metals
Base metals are very common in the earth’s crust, and because of their quantity, they’re inexpensive. Base metals are different from other metals because they corrode or oxidize the most easily. They’re extremely reactive and things like oxygen, water, acids, and being next to a different metal can cause them to corrode. Learn more about why metals rust here.
There are a few different definitions of “base metal”. In mining and economics, the base metals are the metals that don’t fall into any of the other categories like copper, lead, zinc, and nickel.
Ferrous Metals
Ferrous metals means that the metal contains iron. Non-ferrous metals are generally more expensive because they are lower in weight, more conductive, non-magnetic, and resistant to corrosion. Non-ferrous metals include aluminum, copper, lead, nickel, tin, and zinc.
Noble Metals
Noble metals are famous for being resistant to corrosion and oxidation, unlike base metals. They’re usually rare or precious metals. Scientists don’t agree about the exact categorization of every element on the periodic table, but the most commonly agreed on metals that fall under the “noble” category are gold, silver, ruthenium, rhodium, palladium, osmium, iridium, and platinum. Although some precious metals are noble metals, and noble metals are often expensive because of their varied uses (in art, high technology, jewelry), the terms “noble metal” and “precious metal” are not synonymous.
Precious Metals
Precious metals are rare elements that are naturally occurring in the earth’s crust. The most famous are gold and silver, but other precious metals include ruthenium, rhodium, palladium, osmium, iridium, and platinum. Despite being the Earth’s 3rd most common element, and most abundant metal – aluminum was actually considered to be a precious metal for some time. That’s because it was difficult to reliably extract it from its various ores.
Some of Napoleon III’s most important guests were given aluminum cutlery, while the more lowly visitors had to eat with meager silver utensils. The price dropped significantly after 1882 and the invention of new processes for commercial electric generation made extracting aluminum significantly easier.
Heavy Metals
Heavy metals are defined as being very dense. That’s about it! More specific definitions have been proposed, but the scientific community hasn’t settled on one yet. Some heavy metals are notoriously toxic, while others are very important to eat in your diet in trace amounts! Some heavy metals like cadmium, mercury, and lead are extremely poisonous. Metals like iron, cobalt, and zinc on the other hand perform very important functions in the body! You can even buy iron and zinc as supplements at the health food store.
The rest of the heavy metals like gallium, thallium, ruthenium, indium, and silver are pretty harmless. You can find heavy metals in particular in use all over the place! They’re used in golf clubs, cars, antiseptics, self-cleaning ovens, plastics, solar panels, cell phones, computer chips, and even particle accelerators in nuclear power plants!
What is a Metal Alloy?
Alloys on the other hand are man-made materials. You make them by combining a metallic element with something else. Alloys can involve combining a metal with metals, non-metals, or both.
Cast iron is a great example of a non-metal alloy (which is a little misleading, because all alloys have “metal” – it’s referring to the second or added ingredient). The iron is a mixture of iron and carbon. It can range from having about 2-3% carbon. Learn more about cast iron and wrought iron here!
Alloys also sometimes get fun names! Like Alnico, an alloy of iron, aluminum, nickel, cobalt, copper, and/or titanium. Some of their names are an amalgamation of the names of the alloying agents. Other times they just become so popular that they get their own more “every day” sounding name, like wrought iron.
You can truly find alloys everywhere. In fact, they may be more common than their pure “metal” cousins.
You’ll find them in dental fillings (amalgam), guitar pickups (alnico), posing as musical instruments or doorknobs (brass), jewelry (white gold), artwork (bronze statues), in cars and planes (duralumin), on guns (gunmetal), inside electronics (solder), inside nuclear power plants (magnox), holding up buildings (steel), and even on your dining room table (pewter)!
There are more than 160 different known alloys!
Metal Alloy Structures
When metal is magnified through an electron microscope, the atoms appear in a crystalline lattice structure. Also in this structure are alloying agents. Typically, there are two types of alloy structures: substitution alloys and interstitial alloys. Substitution alloys occur if the atoms of an alloying agent replace the atoms of a main metal. Interstitial alloys on the other hand, occur when allots form due to the alloying agents becoming smaller than the main metal.
How are Metal Alloys Made?
There are 3 main methods of creating metal alloys:
- Heating and Melting
- Powder Metallurgy
- Ion Implantation
Heating and Melting
Heating and melting is one of the most commonly used methods for creating alloys. It’s really not that much different from cooking!
The parent metal (the highest % metal in the alloy) is melted down, and any other metals are melted down until they become liquids. Then they’re poured into each other and mixed together, and allowed to cool into something called a “solid solution”. Kind of like a solid metal block equivalent of mixing salt into water until it dissolves.
Powder Metallurgy
Powder metallurgy is very cool, it’s probably the closest thing to alchemy we have today.
First, the parent metal and the alloying agents need to be turned into powders! There are a few main methods for doing this:
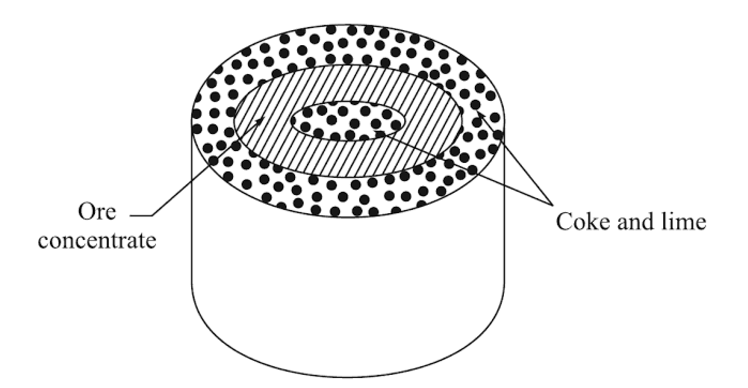
The sponge iron process is the oldest of the powderization techniques. The ore is mixed with something called a coke breeze (which is what’s left of coal after you burn it), and lime in order to make a special sulfur that avoids contamination with the powdered parent metal.
The coke and lime mixture (not like cocktails!) and the ore are then put into a special drum, the coke, and lime sandwiching the ore between it.
Then the drum is superheated in a kiln. The ingredients leave behind a “sponge cake” looking object and a slag. In the following steps, the eventual powder is separated from the slag and crushed into a more uniform “powder” shape.
The powder is then heated, and super compressed into an alloy!
Another way that the parent metals are turned into a powder is by atomization (almost like nuclear power plants use), where molten metal is pushed through a very narrow tube – which makes it high pressure. A gas is injected into the stream of boiling metal exactly as it comes out from this tube, the combination of the pressure, temperature, and the gas molecules separates the atoms of the metal.
The powders are then mixed, and melted together into a “solid solution”!
The iron powder created with the sponge iron process is the cheapest on the global market!
Ion Implementation
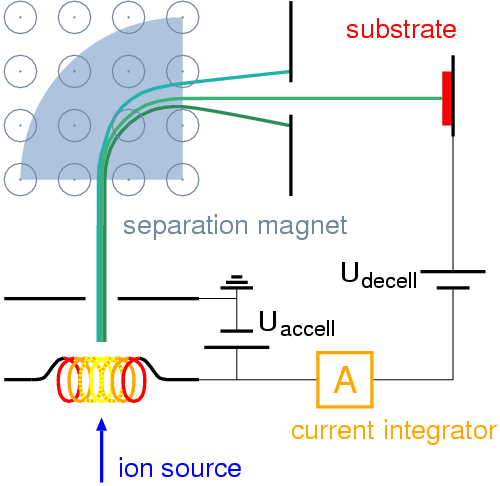
The final common method of creating alloys is ion implantation.
“Ions” come from electricity, so the ion implantation method involves an “ion source” (which just creates electricity basically), an accelerator where the ions are sped up really really fast (friction and fast turning create heat which speeds up molecules), and a target chamber where the ions are tossed after they’re done.
The ion implantation method is really best for creating very small pieces of metal. This is the most common method for creating semiconductors on computer chips.
Here’s an animation of this process:
Metal Supplies and Services at Tampa Steel & Supply
Metal alloys are used in a variety of projects, from residential and commercial construction to the production of cars and more. If you’re working with metal alloys, Tampa Steel and Supply can help. We stock an extensive list of metal products, fabrication supplies, and additional services that can get the ball rolling on your project. To learn more about our products and services, give us a call today or stop by our showroom in Tampa.
Request a Quote Online
Or Call Tampa Steel & Supply at (813) 241-2801